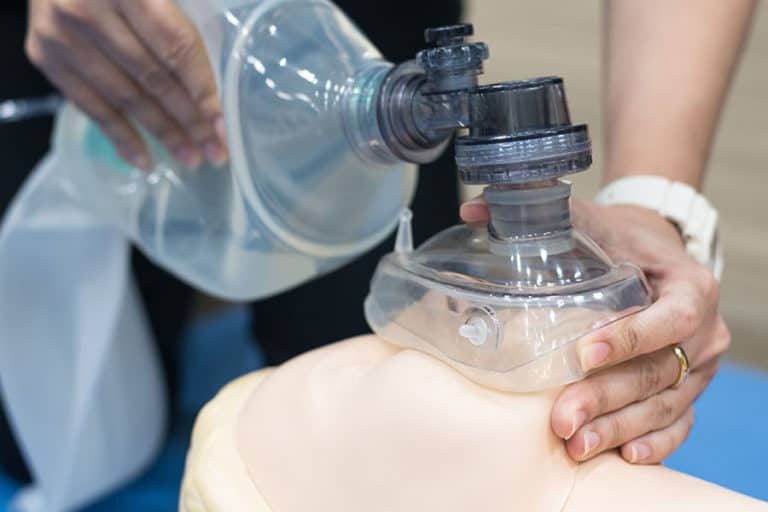
Medical Device Testing
Emmace focuses on the characterisation of medical devices that are in indirect contact with the airways. Both ISO accredited tests (according to ISO 18562) and technical tests are provided to clients in this field. We are also interested to develop new and specially adapted methods jointly together with the client.


Testing of medical devices in in-direct contact with the breathing pathways
Our main focus is on biocompatibility evaluation of medical devices that are included in set-ups that can release particulate matters (particles), volatile organic components, as well as very volatile organic components.
We also have technical methods for characterizing the amount of condensate volumes from the medical devices as well as collection and quantification of isocyanates emitting from medical devices. More information is found under each specific analysis below:
- ISO 18562-2 Particulate matter (PM)
- ISO 18562-3 Volatile organic components (VOC)
- ISO 18562-4 Condensate testing
- ISO 27427 Nebulizer testing
- ISO 9360 Moisture Loss, to be updated
- ISO 13320 Laser diffraction testing